|
May 2023
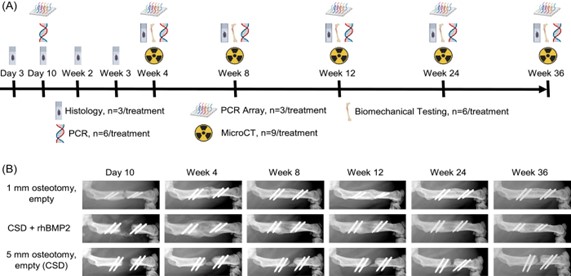
ISFR Selected Publications From This Quarter (ISFR Members Highlighted in Green) Segmental defect healing in the presence or absence of recombinant human BMP2: Novel insights from a rat model Joseph A. Panos, Michael J. Coenen, Christopher V. Nagelli, Erin B. McGlinch, Aysegul Atasoy-Zeybek, Consuelo Lopez De Padilla, Rodolfo E. De la Vega, Christopher H. Evans (Journal of Orthopaedic Research, February 27, 2023) https://onlinelibrary.wiley.com/doi/10.1002/jor.25530 Minced muscle autografting improves bone healing but not muscle function in a porcine composite injury model Todd O. McKinley, Roman N. Natoli, Naveena B. Janakiram, Stuart J. Warden, Robyn K. Fuchs, Zachary Gunderson, Nichlaus Diggins, Seungyup Sun, George Kolettis, Stephen M. Goldman, Christopher L. Dearth, Stephen Mendenhall, Caio Staut, Melissa A. Kacena, Benjamin T. Corona (Journal of Orthopaedic Research, March 16, 2023) https://onlinelibrary.wiley.com/doi/10.1002/jor.25551 Reduced angiogenesis and delayed endochondral ossification in CD163−/− mice highlights a role of M2 macrophages during bone fracture repair Youliang Ren, Shiyang Zhang, Jason Weeks, Javier Rangel Moreno, Bin He, Thomas Xue, Joshua Rainbolt, Yugo Morita, Ye Shu, Yuting Liu, Stephen L. Kates, Edward M. Schwarz, Chao Xie (Journal of Orthopaedic Research, March 27, 2023) https://pubmed.ncbi.nlm.nih.gov/36970754/ Postnatal Osterix but not DMP1 lineage cells significantly contribute to intramembranous ossification in three preclinical models of bone injury Evan G Buettmann, Susumu Yoneda, Pei Hu, Jennifer A McKenzie, Matthew J Silva (Frontiers in Physiology, January 4, 2023) https://pubmed.ncbi.nlm.nih.gov/36685200/ Altered early immune response after fracture and traumatic brain injury Melanie Haffner-Luntzer, Birte Weber, Kazuhito Morioka, Ina Lackner, Verena Fischer, Chelsea Bahney, Anita Ignatius, Miriam Kalbitz, Ralph Marcucio, Theodore Miclau (Frontiers in Immunology, January 25, 2023) https://pubmed.ncbi.nlm.nih.gov/36761764/ Wnt-associated adult stem cell marker Lgr6 is required for osteogenesis and fracture healing Laura Doherty, Matthew Wan, Anna Peterson, Daniel W Youngstrom, Justin S King, Ivo Kalajzic, Kurt D Hankenson, Archana Sanjay (Bone, April 2023) https://pubmed.ncbi.nlm.nih.gov/36708855/ Section Member Journal Article Review Segmental defect healing in the presence or absence of recombinant human BMP2: Novel insights from a rat model Joseph A. Panos, Michael J. Coenen, Christopher V. Nagelli, Erin B. McGlinch, Aysegul Atasoy-Zeybek, Consuelo Lopez De Padilla, Rodolfo E. De la Vega, Christopher H. Evans (Journal of Orthopaedic Research, February 27, 2023) https://pubmed.ncbi.nlm.nih.gov/36850029/ Despite bone’s innate regenerative capacity, large segmental defects fail to heal and, therefore, require clinical treatment to stimulate healing. Ideally, treatments would stimulate endochondral ossification to produce high-quality and mechanically-stable bridging of the two bone ends. Recombinant, human bone morphogenetic protein-2 (rhBMP-2) is a widely used osteoinductive therapeutic to treat segmental defects. While high doses of rhBMP2 increase bone healing, the bone formed is of poorer quality when compared to the bone formed in a sub-critical defect. However, the underlying biological basis for this poor bone quality is unknown. Joseph Panos and colleagues aimed to understand the biological underpinnings that produce lower quality bone in rhBMP2 stimulated healing in a rat segmental defect model. In order to understand how rhBMP2 affects healing, they used both a critical sized defect (5mm), either with or without rhBMP2 delivered in an absorbable collagen sponge, and a non-stimulated sub-critical defect model (1mm). The 1mm sub-critical defect heals naturally through endochondral ossification, acting as a “positive control” group with well-defined healing time points that were used to understand how the rhBMP2 affected the healing progression. After creating the defects, they evaluated healing progression with histology, microCT, and mechanical testing. The non-critical defect progressed through the typical healing cascade over 12 weeks., beginning with hematoma formation, soft cartilage formation, and ending by transition into a hard callus that bridged the defect. By 36 weeks, inner and outer marrow cavities were formed and mechanical strength was restored back to non-injured levels. The non-stimulated critical sized defect formed a hematoma and endochondral ossification capped the two bone ends. However, healing never progressed to fully bridge the gap. In rhBMP2-treated critical sized defects, they found that cell infiltration was significantly impaired due to the collagen scaffold. Interestingly, Safarin O staining of rhBMP2-treated defects showed little cartilage formation, reflecting that rhBMP2 likely is not stimulating endochondral ossification. However, rhBMP2-treated defects bridged earlier and with a larger callus than the sub-critical defects. This early bridging was associated with increased mechanical integrity when compared to the sub-critical defect; but, by 36 weeks, rhBMP2-treated bones were less strong than naturally healing sub-critical defect bones. To understand the biological basis for these differences in healing, they profiled the kinetics of immune and osteogenic genes. Compared to naturally healing sub-critical defects, which had immune cytokine expression through 12 weeks, rhBMP2 treatment caused sustained expression through 24 weeks. rhBMP2 treatments also caused an earlier increase in BMP7 expression and sustained Phex expression, which quickly peaked and decreased starting at Day 10 in sub-critical defects. Most interestingly, chondrocyte markers Col2a1 and Col10a1 were 100-fold higher in sub-critical defects than in rhBMP2-treated defects, further reinforcing that endochondral ossification is likely not a major healing pathway stimulated by rhBMP2. In all, Panos and colleagues concluded that it is likely due to differences in ossification programs that lead to the distinct mechanical quality differences in naturally and rhBMP2-stimulated healing. Natural healing in sub-critical defects occurs through endochondral ossification, whereas rhBMP2 stimulation in a critical sized defect predominantly drove intramembranous ossification. While this intramembranous ossification resulted in early defect bridging and a quick increase in mechanical stability, over time, the bone quality decreases so that rhBMP2-stimulated defects were significantly weaker. These differences may be due to changes in the early immune response, which may function to inhibit chondrogenic differentiation in rhBMP2-stimulated healing. Overall, though rhBMP2 treatment is a powerful tool to help bridge defects that would not naturally heal without intervention, identifying new therapeutic targets that promote or accelerate endochondral ossification may produce superior bone that restores mechanical integrity better than rhBMP2.
|


 Evan Buettmann, PhD
Evan Buettmann, PhD Nicole Gould, PhD.
Nicole Gould, PhD. Madhura Nijsure
Madhura Nijsure Augustine M. Saiz, MD
Augustine M. Saiz, MD Andrea Alford, PhD
Andrea Alford, PhD Rodolfo E. de la Vega, MD
Rodolfo E. de la Vega, MD